Taking a deeper look into the chemistry of water treatment polymers, more specifically water soluble polymers.
Water treatment polymers chemistry
Last week’s blog post we took a look into flocculants and more specifically “what are flocculants, what do they look like and how do they work?”
Flocculants fall into the water soluble polymer category. Polymers are long chained molecules composed of basic building blocks called monomers. The monomers are reacted end to end to make a molecular ‘rope’ so to speak that can be either linear or branched where shorter ‘ropes’ are attached to the longer ropes. These shorter polymers can even connect together other long chained polymers, but when this becomes extensive, the polymer becomes much less water soluble and fall into the water absorptive class of ‘water swelling’ polymers.
This week we will build on the last blog discussion and focus on water soluble polymers and their unique characteristics.
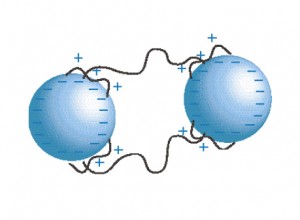
Polymer Bridging Flocculant – Illustration provided by SNF Holding Company
Water treatment polymers are used to ‘lasso’ individual particles into larger aggregates. In settling type solids-liquid (S/L) separation operations such as thickeners and clarifiers, these much larger floccules settle faster in a liquid which has viscosity that impedes a fine-small particle’s decent and because of the particle aggregate increased macroscale can overcome eddy currents which can reduce settling rate. Water treatment polymers enhance settling rates and consequently S/L unit operation capacity. In other types of operations such as filtering, dissolved air flotation, centrifuges and the like the flocculated particles open channels for the liquid to more readily drain or be forced through the floccules. Very fine particles are very difficult to remove in S/L operations unless these are attached to larger, heavier particles or particle aggregates; failure to effectively capture these fine particles causes clarity issues and reduces capture rates.
In this regard, water treatment polymer chain length is an important characteristic. Depending on the mineral and sludge subtrates and particle sizes treated, selecting the optimum water treatment polymer chain length has a significant impact on effective flocculation of the broad range of particle sizes and composition present into floccule aggregates. In many cases, particularly with organic sludges, the degree of polymer crosslinking can be an important organic sludge treatment variable related to satisfying surface ‘cationic charge demand’ more effectively.
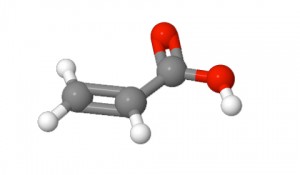
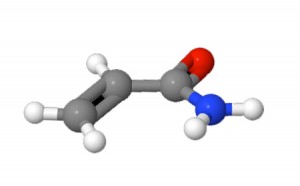
The other main polymer characteristic is flocculant ionic charge type and % charge. Flocculant ionic charge is imparted by the specific monomer chemistries used and their proportions. While several types of monomers are used, most of the common flocculants are composed of one of the following monomers from which the common nonionic, anionic and cationic flocculant classes are manufactured:
|
|
|
|
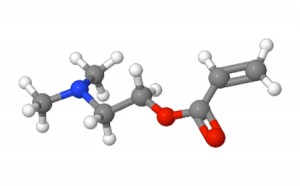 2-(Dimethylamino)ethyl acrylate – illustration provided by ChemSpider |
|
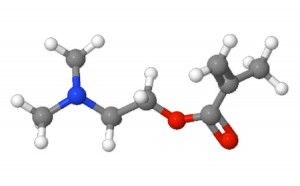 2-(Dimethylamino)ethyl methacrylate – illustration provided by ChemSpider |
|
Manufacturing the flocculant ionic type and charge needed is just a matter of blending the appropriate type and proportion of each monomer required. For instance, in the manufacture of a 20% anionic charged polyacrylamide, 20% anionic acrylic acid anionic monomer is blended with 80% of polyacrylamide nonionic monomer which are reacted into chained polymers. In the polymeric reactions the acrylic acid monomers distribute between the polyacrylamide monomers in the chain depending on manufacturing conditions, probabilities and reaction thermodynamics/kinetics.
The term % charge can refer to either the amount of anionic or cationic monomer in the polymer either as proportions based on % mole or % of molecular weight of the total polymer molecular weight. As a general custom, most manufacturers use % mole charge. This is an important distinction each type of monomer has a different molecular weight. Using % mole charge is straight forward.
Considering all the potential combinations of chain length, degree of branching, ionic charge type and % charge, the spectrum of possible polymer chemistries is very large. Fortunately only a subset of the polymer chemistry combinations is required in S/L applications. The challenge is selecting the product with the optimum chemistry combination for each application which will typically exhibit varying solids response characteristics. When the correct chain length and flocculant charge type/level are selected for a slurry and sludge system, the separated water clarities are high and the flocculant consumption rates are minimized. Discussion of flocculant selection will be further discussed in a future blog
Download Product Brochure Download PDF Version
Contact Zeroday today at 1-503-582-9067 to discuss your water soluble polymer needs.
Categories: Chemicals