Key factors you should know about mixing dry flocculants.
Mixing dry flocculants can be easy or a great, messy challenge if flocculant chemistry properties and physical characteristics are not accommodated. As explained in previous blogs, dry flocculants are very water soluble, are long chained, have very high molecular weight and the dry flocculant granules are sized to maximize the dissolution rate but are not so fine as to create dust issues.
Dry flocculant characteristics that must be accommodated when mixing-dissolving include:
- Even slightly wetted flocculant particles are sticky.
- Wetted flocculant particles will stick to equipment surfaces and accumulate into masses that can disrupt the mixing process.
- While flocculant particles will hydrate to a clear gel in a few minutes, the flocculant molecules actually dissolve from the flocculant mass surface inward so dissolution rate is surface area driven and large clumps of flocculant particle aggregates dissolve much slower than the individual particles.
- As a macro molecule, long chained polymers dissolve from the flocculant particle surface relatively slowly.
- Dissolution rate is affected and is a function of water chemistry, water temperature, mixing speed, flocculant molecular weight, ionic charge type and % charge.
Flocculant dissolution begins even when the flocculant particles are only partially wetted. Partially dissolved flocculant particles will stick together and increase mixing time, build up on equipment surfaces and impede the mixing process, plug the system entirely or cause slipping safety issues. Consequently mixing system design must incorporate provisions to minimize the occurrence of flocculant buildup.
Because wetted flocculant particles are sticky, it is crucial to individually wet and mix the flocculant particles in a manner to prevent these from aggregating into particle masses. Flocculants are hygroscopic and consequently can absorb sufficient moisture from the air causing minor surface dissolution and flocculant particle clumping even in a hopper or the supposedly water proof sack.

Result of absorbed moister from air by flocculants
If flocculant clumps occur, these masses will hydrolyze fairly quickly into clear gelatinous masses, and dissolve very slowly due to their significantly reduced surface area in comparison to the total surface area of the individual particles that make up the gelatinous mass. These masses are called ‘fish eyes’ because their presence is only detectable by light refraction through clear gelatinous masses which resemble actual clear fish eyes in water.
The flocculant molecules coiled on the flocculant particles initially hydrate which allows these to fully dissolve into the bulk water phase. Stretched out straight, these are very long molecules; the author once calculated if a typical flocculant molecule is magnified to 1 inch (25 mm) diameter, the flocculant chain would be over 1.25 miles (2 km) long! The flocculant molecules do not exist as extended straight chains in water solution due to the very low probability of that happening.
Each monomer bonds in the polymer chain are basically independent freely moving and twisting joints that move randomly. Brownian motion, water currents, etc. cause the polymer to twist and turn and help the dissolved polymer chain extend outward, however, because there are thousands of independently flexing and moving joints the molecule never actually fully extends straight as being a statistical improbability. Consequently flocculant molecules in solution generally are coiled to at least some extent.
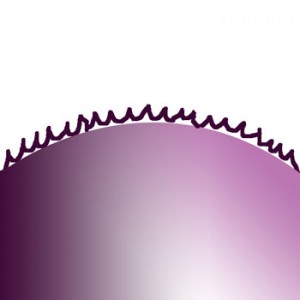 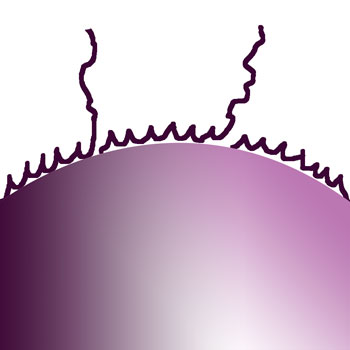 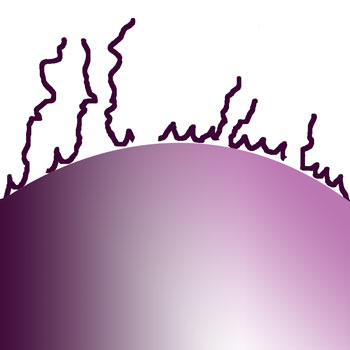 |
Stages of dry flocculants dissolving from surface |
These dissolved long chained molecules cause viscosity because through interaction with a moving object in the flocculant solution, a ‘drag’ and resistance is imparted by the solution on the object. The more extended the molecule, the greater the viscosity. Solutions of higher ionically charged polymers are more viscous because the identical charges distributed throughout the molecule backbone repel each other like the north poles of two magnets repel each other causing more polymer molecule extension. The charges can be either anionic or cationic but the result is these charges induce the polymers to be straighter than low charged flocculant molecules.
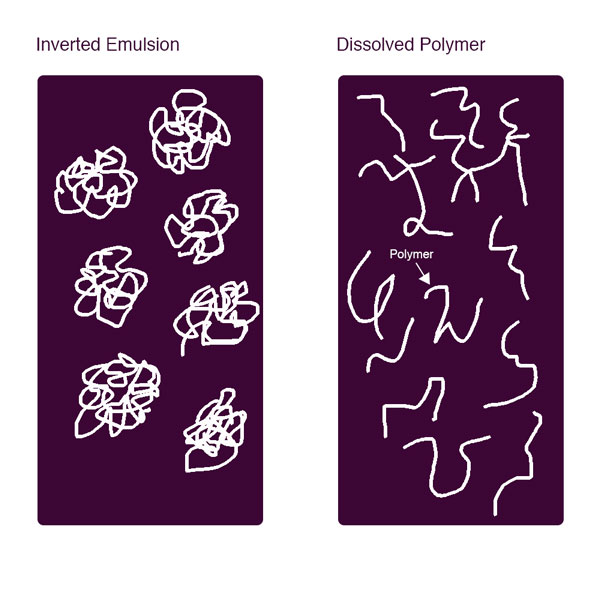
Water chemistry and temperature conditions impact flocculant dissolution rates. For instance, flocculants dissolve faster in warmer water since dissolution rates are inversely proportional to temperature and solution viscosity increases inversely with temperature. Also flocculant solutions made with high ionic strength water dissolve faster but solutions will have lower viscosity because, since the water ions act as strong counter ions to flocculant charged sites, the flocculants in effect will be lower charged and consequently more coiled.
Download Product Brochure Download PDF Version
Contact Zeroday today at 1-503-582-9067 to discuss your water soluble polymer needs.
Categories: Flocculants

